-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Put all BD® AbSeq Reagents to be pooled into a Latch Rack for 500 µL Tubes (Thermo Fisher Scientific Cat. No. 4900). Arrange the tubes so that they can be easily uncapped and re-capped with an 8-Channel Screw Cap Tube Capper (Thermo Fisher Scientific Cat. No. 4105MAT) and the reagents aliquoted with a multi-channel pipette.
BD® AbSeq tubes should be centrifuged for ≥ 30 seconds at 400 × g to ensure removal of any content in the cap/tube threads prior to the first opening.
Product Notices
- This reagent has been pre-diluted for use at the recommended volume per test. Typical use is 2 µl for 1 × 10^6 cells in a 200-µl staining reaction.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Illumina is a trademark of Illumina, Inc.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Please refer to bd.com/genomics-resources for technical protocols.
- For U.S. patents that may apply, see bd.com/patents.
Companion Products
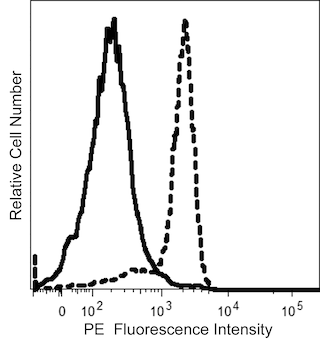





The 563 monoclonal antibody specifically binds to CD34. CD34 is a single-chain 105-120 kDa heavily O-glycosylated transmembrane glycoprotein expressed on hematopoietic progenitor cells, vascular endothelium and some tissue fibroblasts. The intracellular chain of the CD34 antigen is a target for phosphorylation by activated protein kinase C suggesting that CD34 may play a role in signal transduction. CD34 may play a role in adhesion of specific antigens to endothelium. Clone 563 belongs to the class III epitope; it is resistant to neuraminidase, chymopapain and glycoprotease. This antibody is able to block the binding of other CD34 mAbs like 581.
Development References (11)
-
Egeland T, Tjonnfjord G, Steen R, Gaudernack G, Thorsby E. Positive selection of bone marrow-derived CD34 positive cells for possible stem cell transplantation. Transplant Proc. 1993; 25(1):1261-1263. (Biology). View Reference
-
Gaudernack G, Egeland T. Epitope mapping of 33 CD34 mAb, including the Fith Workshop Panel. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:861-864.
-
Greaves MF, Titley I, Colman SM, et al. CD34 cluster workshop report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:840-846.
-
Kishimoto T. Tadamitsu Kishimoto .. et al., ed. Leucocyte typing VI : white cell differentiation antigens : proceedings of the sixth international workshop and conference held in Kobe, Japan, 10-14 November 1996. New York: Garland Pub.; 1997.
-
Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:1-1182.
-
Lin G-X, Yang X, Hollemweguer E, et al. Cross-reactivity of CD antibodies in eight animal species. In: Mason D. David Mason .. et al., ed. Leucocyte typing VII : white cell differentiation antigens : proceedings of the Seventh International Workshop and Conference held in Harrogate, United Kingdom. Oxford: Oxford University Press; 2002:519-523.
-
Nishio H, Tada J, Hashiyama N, Hirn J, Ingles-Esteven J, Suda T. CD34. 1999. Available: http://mpr.nci.nih.gov/prow/guide/968267813_g.htm 2006, February 8.
-
Owens MA, Loken MR. Peripheral blood stem cell quantitation. In: Owens MA, Loken MR. Flow Cytometry Principles for Clinical Laboratory Practice. New York: John Wiley & Sons; 1995:128.
-
Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995.
-
Steen R, Egeland T. CD34 molecule epitope distribution on cells of haematopoietic origin.. Leuk Lymphoma. 1998; 30(1-2):23-30. (Clone-specific: Flow cytometry). View Reference
-
Strawn WB, Ferrario CM. Angiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeys.. Atherosclerosis. 2008; 196(2):624-32. (Clone-specific: Flow cytometry, Fluorescence activated cell sorting). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.