-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

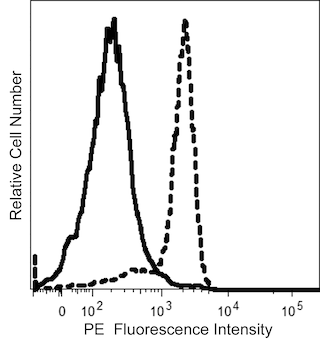
Multiparameter flow cytometric analysis using BD OptiBuild™ R718 Mouse Anti-Human CD34 antibody (Cat. No. 752176; Right Plot) on Human peripheral blood mononuclear cells, with corresponding IgG Isotype Control (Cat. No. 566928; Left Plot). Flow cytometry was performed using a BD LSRFortessa™ X-20 Flow Cytometer System.
.png)

BD OptiBuild™ R718 Mouse Anti-Human CD34
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
- For U.S. patents that may apply, see bd.com/patents.
Companion Products



.png?imwidth=320)
The 8G12 monoclonal antibody specifically recognizes CD34, a 105-120 kDa single-chain type I transmembrane glycoprotein. The 8G12 antibody recognizes an epitope on CD34 distinct from the one recognized by clone My10. CD34 is expressed on immature hematopoietic precursor cells and all hematopoietic colony-forming cells in bone marrow and blood, including unipotent (CFU-GM, BFU-E) and pluripotent progenitors (CFU-GEMM, CFU-Mix, and CFUBlast). The CD34 antigen is a differentiation stage-specific leucocyte antigen. Terminal deoxynucleotidyl transferase-positive B- and T-lymphoid precursors in normal bone marrow are CD34+. The CD34 antigen is present on early myeloid cells that express the CD33 antigen but lack the CD14 and CD15 antigens and on early erythroid cells that express the CD71 antigen and dimly express the CD45 antigen. The CD34 antigen is also found on capillary endothelial cells and approximately 1% of human thymocytes. Normal peripheral blood lymphocytes, monocytes, granulocytes, and platelets do not express CD34. CD34 density is highest on early hematopoietic progenitor cells and decreases as cells mature. The antigen is absent on fully differentiated hematopoietic cells. Uncommitted CD34+ progenitor cells are CD38- and lack lineage-specific antigens such as CD71, CD33, CD10, and CD5, while CD34+ cells that are lineage-committed express the CD38 antigen in high density. Most CD34+ cells reciprocally express either the CD45RO or CD45RA antigens, with the CD45RO+ population being the more primitive. Approximately 60% of acute B-lymphoid leukemias and acute myeloid leukemias (AML) and 1% to 5% of acute T-lymphoid leukemias express CD34. CD34 is not expressed on chronic lymphoid leukemias or lymphomas.

Development References (17)
-
Lansdorp PM, Dougherty GJ, Humphries RK. CD34 epitopes. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:826-827.
-
Andrews RG, Singer JW, Bernstein ID. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties.. J Exp Med. 1989; 169(5):1721-31. (Biology). View Reference
-
Brocklebank AM, Sparrow RL. Enumeration of CD34+ cells in cord blood: a variation on a single-platform flow cytometric method based on the ISHAGE gating strategy.. Cytometry. 2001; 46(4):254-61. (Biology). View Reference
-
Gore SD, Kastan MB, Civin CI. Normal human bone marrow precursors that express terminal deoxynucleotidyl transferase include T-cell precursors and possible lymphoid stem cells.. Blood. 1991; 77(8):1681-90. (Biology). View Reference
-
Greaves MF, Titley I, Colman SM, et al. CD34 cluster workshop report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:840-846.
-
Hurwitz CA, Loken MR, Graham ML, et al. Asynchronous antigen expression in B lineage acute lymphoblastic leukemia.. Blood. 1988; 72(1):299-307. (Biology). View Reference
-
Kurtzberg J, Denning SM, Nycum LM, Singer KH, Haynes BF. Immature human thymocytes can be driven to differentiate into nonlymphoid lineages by cytokines from thymic epithelial cells.. Proc Natl Acad Sci USA. 1989; 86(19):7575-9. (Biology). View Reference
-
Lansdorp PM, Sutherland HJ, Eaves CJ. Selective expression of CD45 isoforms on functional subpopulations of CD34+ hemopoietic cells from human bone marrow.. J Exp Med. 1990; 172(1):363-6. (Clone-specific: Flow cytometry). View Reference
-
Lanza F, Moretti S, Papa S, Malavasi F, Castoldi G. Report on the Fifth International Workshop on Human Leukocyte Differentiation Antigens, Boston, November 3-7, 1993.. Haematologica. 79(4):374-86. (Clone-specific: Flow cytometry). View Reference
-
Leary AG, Strauss LC, Civin CI, Ogawa M. Disparate differentiation in hemopoietic colonies derived from human paired progenitors.. Blood. 1985; 66(2):327-32. (Biology). View Reference
-
Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987; 70(5):1316-1324. (Biology). View Reference
-
Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow: I. Normal erythroid development.. Blood. 1987; 69(1):255-63. (Biology). View Reference
-
Peschel C, Köller U. Cluster report: CD34. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:817-818. View Reference
-
Ryan D, Kossover S, Mitchell S, Frantz C, Hennessy L, Cohen H. Subpopulations of common acute lymphoblastic leukemia antigen-positive lymphoid cells in normal bone marrow identified by hematopoietic differentiation antigens.. Blood. 1986; 68(2):417-25. (Biology). View Reference
-
Siena S, Bregni M, Brando B, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients.. Blood. 1991; 77(2):400-9. (Clone-specific: Flow cytometry). View Reference
-
Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991; 77(6):1218-1227. (Clone-specific: Flow cytometry, Fluorescence activated cell sorting). View Reference
-
Terstappen LW, Safford M, Könemann S, et al. Flow cytometric characterization of acute myeloid leukemia. Part II. Phenotypic heterogeneity at diagnosis.. Leukemia. 1992; 6(1):70-80. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.