-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
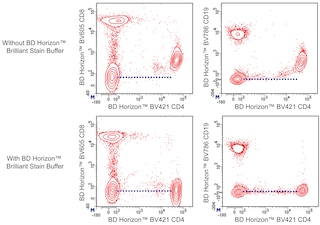
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Violet 711 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
- Cy is a trademark of GE Healthcare.
- Alexa Fluor® is a registered trademark of Life Technologies Corporation.
Companion Products
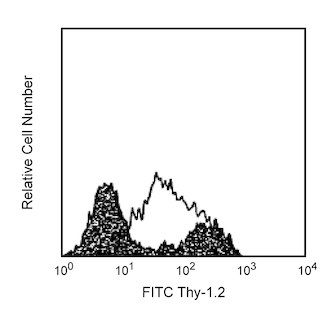



The 927 monoclonal antibody specifically recognizes CD317 which is also known as Bone marrow stromal antigen 2 (BST2) or Plasmacytoid Dendritic Cell Ag-1 (PDCA-1). CD317 (BST2) is a type II transmembrane glycoprotein that is encoded by Bst2. It is highly expressed on naïve plasmacytoid dendritic cells (pDC) which comprise very small populations within lymphoid and nonlymphoid tissues. pDC are characteristically early responders to viruses through Toll-like receptors (TLR), TLR7 and TLR9. Activated pDC can produce large amounts of proinflammatory cytokines including type I interferons, Interferon-alpha (IFN-α) and Interferon-beta (IFN-β), and are also known as Type I IFN producing cells (IPC). CD317 (BST2) expression can be upregulated on a variety of other cell types following stimulation with type I IFNs and IFN gamma including T cells, B cells, plasma cells, NK cells, CD8+ and CD8- dendritic cells, and cell lines. CD317 (BST2) may play a role in sorting proteins, eg, cytokines, from the Golgi apparatus and the plasma membrane.
The antibody was conjugated to BD Horizon™ BV711 which is part of the BD Horizon Brilliant™ Violet family of dyes. This dye is a tandem fluorochrome of BD Horizon BV421 with an Ex Max of 405-nm and an acceptor dye with an Em Max at 711-nm. BD Horizon BV711 can be excited by the violet laser and detected in a filter used to detect Cy™5.5 / Alexa Fluor® 700-like dyes (eg, 712/20-nm filter). Due to the excitation and emission characteristics of the acceptor dye, there may be moderate spillover into the Alexa Fluor® 700 and PerCP-Cy5.5 detectors. However, the spillover can be corrected through compensation as with any other dye combination.

Development References (2)
-
Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation.. J Immunol. 2006; 177(5):3260-5. (Immunogen: Flow cytometry, Fluorescence microscopy, Immunofluorescence). View Reference
-
Ohtomo T, Sugamata Y, Ozaki Y, et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999; 258(3):583-591. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.