-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
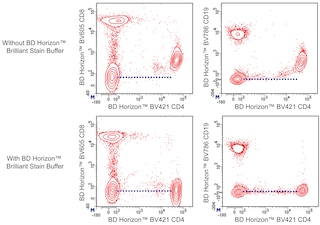
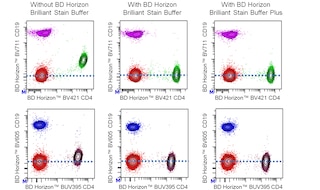
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime BD Horizon Brilliant dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Note: When using high concentrations of antibody, background binding of this dye to erythroid cell subsets (mature erythrocytes and precursors) has been observed. For researchers studying these cell populations, or in cases where light scatter gating does not adequately exclude these Cells from the analysis, this background may be an important factor to consider when selecting reagents for panel(s).
Product Notices
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Ultraviolet 661 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,575,303; 8,354,239.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- For U.S. patents that may apply, see bd.com/patents.
Companion Products
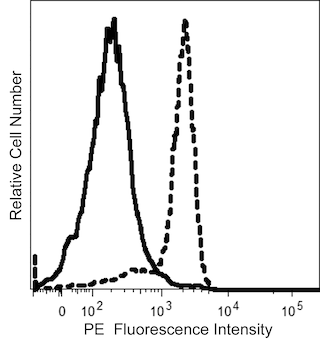



The 563 monoclonal antibody specifically binds to CD34. CD34 is a single-chain 105-120 kDa heavily O-glycosylated transmembrane glycoprotein expressed on hematopoietic progenitor cells, vascular endothelium and some tissue fibroblasts. The intracellular chain of the CD34 antigen is a target for phosphorylation by activated protein kinase C suggesting that CD34 may play a role in signal transduction. CD34 may play a role in adhesion of specific antigens to endothelium. Clone 563 belongs to the class III epitope; it is resistant to neuraminidase, chymopapain and glycoprotease. This antibody is able to block the binding of other CD34 mAbs like 581.
Development References (11)
-
Egeland T, Tjonnfjord G, Steen R, Gaudernack G, Thorsby E. Positive selection of bone marrow-derived CD34 positive cells for possible stem cell transplantation. Transplant Proc. 1993; 25(1):1261-1263. (Biology). View Reference
-
Gaudernack G, Egeland T. Epitope mapping of 33 CD34 mAb, including the Fith Workshop Panel. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:861-864.
-
Greaves MF, Titley I, Colman SM, et al. CD34 cluster workshop report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:840-846.
-
Kishimoto T. Tadamitsu Kishimoto .. et al., ed. Leucocyte typing VI : white cell differentiation antigens : proceedings of the sixth international workshop and conference held in Kobe, Japan, 10-14 November 1996. New York: Garland Pub.; 1997.
-
Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:1-1182.
-
Lin G-X, Yang X, Hollemweguer E, et al. Cross-reactivity of CD antibodies in eight animal species. In: Mason D. David Mason .. et al., ed. Leucocyte typing VII : white cell differentiation antigens : proceedings of the Seventh International Workshop and Conference held in Harrogate, United Kingdom. Oxford: Oxford University Press; 2002:519-523.
-
Nishio H, Tada J, Hashiyama N, Hirn J, Ingles-Esteven J, Suda T. CD34. 1999. Available: http://mpr.nci.nih.gov/prow/guide/968267813_g.htm 2006, February 8.
-
Owens MA, Loken MR. Peripheral blood stem cell quantitation. In: Owens MA, Loken MR. Flow Cytometry Principles for Clinical Laboratory Practice. New York: John Wiley & Sons; 1995:128.
-
Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995.
-
Steen R, Egeland T. CD34 molecule epitope distribution on cells of haematopoietic origin.. Leuk Lymphoma. 1998; 30(1-2):23-30. (Clone-specific: Flow cytometry). View Reference
-
Strawn WB, Ferrario CM. Angiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeys.. Atherosclerosis. 2008; 196(2):624-32. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.