-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




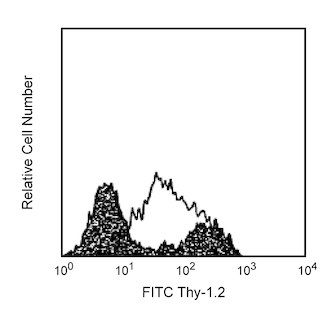
Flow cytometric analysis of mouse CD62L expression - Staining comparisons between BD Horizon™ BB515- and FITC-conjugated antibodies. Left Panel - CD62L expression on mouse bone marrow cells. Mouse bone marrow cells were untreated (Left Plot) or cultured (1 hr) with Phorbol 12-Myristate 13-Acetate (PMA; Middle Plot). The cells were preincubated with Purified Rat Anti-Mouse CD16/CD32 antibody (Mouse BD Fc Block™) (Cat. No. 553141/553142). They were then stained with either BD Horizon BB515 Rat IgG2a, κ Isotype Control (Cat. No. 564418; dashed line histogram) or BD Horizon BB515 Rat Anti-Mouse CD62L antibody (Cat. No. 565261; bold solid line histogram). Alternatively, untreated cells (Right Plot) were stained with FITC Anti-Mouse CD62L antibody (Cat. No. 553150/561917; thin solid line histogram). Overlaid histograms are shown to facilitate staining comparisons between: BB515 Anti-CD62L antibody versus its Ig Isotype Control (Left Plot), and BB515 Anti-CD62L antibody versus FITC Anti-CD62L antibody (Right Plot). The fluorescence histograms showing CD62L expression (or Ig Isotype control staining) were derived from gated events with the forward and side light-scatter characteristics of viable cells using a BD™ LSR II Flow Cytometer System. Right Panel - CD62L expression on mouse splenocytes. Mouse splenic leucocytes were preincubated with Purified Rat Anti-Mouse CD16/CD32 antibody (Mouse BD Fc Block™). The cells were then stained with APC Hamster Anti-Mouse CD3e antibody (Cat. No. 553066/561826) and either BD Horizon BB515 Rat IgG2a, κ Isotype Control (Left Plot), BD Horizon BB515 Rat Anti-Mouse CD62L antibody (Middle Plot), or FITC Anti-Mouse CD62L antibody (Right Plot). Two-color flow cytometric contour plots showing the correlated expression of CD62L (or Ig Isotype control staining) versus CD3e were derived from gated events with the light-scatter characteristics of viable leucocytes using a BD™ LSR II Flow Cytometer System.


BD Horizon™ BB515 Rat Anti-Mouse CD62L

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and CompBead to ensure that BD Comp beads are appropriate for your specific cellular application.
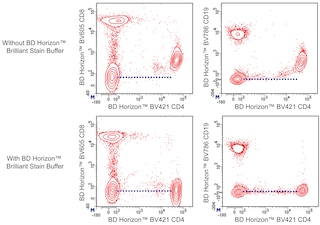
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
For optimal results, it is recommended to perform 2 washes after staining with antibodies. Cells may be prepared, stained with antibodies and washed twice with wash buffer per established protocols for immunofluorescence staining, prior to acquisition on a flow cytometer. Performing fewer than the recommended wash steps may lead to increased spread of the negative population.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




The MEL-14 monoclonal antibody specifically binds to CD62L (L-selectin), a 95 kDa (on neutrophils) or 74 kDa (on lymphocytes) receptor with lectin-like and Epidermal Growth Factor-like domains. In the mouse, L-selectin is detected on most thymocytes, with the highest levels of expression on an immunocompetent subset and a population of dividing progenitor cells, and on peripheral leukocytes, including subsets of B and T lymphocytes, neutrophils, monocytes, and eosinophils. This member of the selectin adhesion molecule family appears to be required for lymphocyte homing to peripheral lymph nodes and to contribute to neutrophil emigration at inflammatory sites. L-selectin is rapidly shed from lymphocytes and neutrophils upon cellular activation; metalloproteinases may mediate the release of CD62L ectodomains from the cell surface. The level of CD62L expression, along with other markers, distinguishes naive, effector, and memory T cells. L-selectin binds to sialytaed oligosaccharide determinants on high endothelial venules (HEV) in peripheral lymph nodes. In vitro studies have demonstrated that CD34, GlyCAM-1, and MAdCAM-1, all recognized by mAb MECA-79 (anti-mouse PNAd Carbohydrate Epitope, Cat. No. 553863), may be ligands for CD62L. MEL-14 mAb blocks in vitro binding of lymphocytes to peripheral lymph node HEV and inhibits in vivo lymphocyte extravasation into peripheral lymph nodes and late stages of leukocyte rolling.
The antibody was conjugated to BD Horizon BB515 which is part of the BD Horizon Brilliant™ Blue family of dyes. With an Ex Max near 490 nm and an Em Max near 515 nm, BD Horizon BB515 can be excited by the blue laser (488 nm) laser and detected with a 530/30 nm filter. This dye has been exclusively developed by BD Biosciences and is up to seven times brighter than FITC with less spillover into the PE channel. Due to similar excitation and emission properties, BB515, FITC, and Alexa Fluor® 488 cannot be used simultaneously. It is not recommended to use BB515 in cocktails that include Streptavidin conjugates as it may cause high background.

Development References (15)
-
Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. The age-associated increase in IFN-γ synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993; 151(2):575-587. (Clone-specific: Flow cytometry). View Reference
-
Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983; 304(5921):30-34. (Immunogen: Blocking, Flow cytometry, Immunoaffinity chromatography, Immunoprecipitation). View Reference
-
Iwabuchi K, Ohgama J, Ogasawara K, et al. Distribution of MEL-14+ cells in various lymphoid tissues. Immunobiology. 1991; 182(2):161-173. (Clone-specific: Cytotoxicity). View Reference
-
Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J Immunol. 1988; 141(12):4110-4117. (Clone-specific: Flow cytometry). View Reference
-
Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989; 245(4923):1238-1241. (Clone-specific: Immunohistochemistry). View Reference
-
Lewinsohn DM, Bargatze RF, Butcher EC. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987; 138(12):4313-4321. (Clone-specific: Blocking, Immunoprecipitation). View Reference
-
Ley K, Bullard DC, Arbones ML, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995; 181(2):669-675. (Clone-specific: Blocking). View Reference
-
Mobley JL, Dailey MO. Regulation of adhesion molecule expression by CD8 T cells in vivo. I. Differential regulation of gp90MEL-14 (LECAM-1), Pgp-1, LFA-1, and VLA-4 alpha during the differentiation of cytotoxic T lymphocytes induced by allografts. J Immunol. 1992; 148(8):2348-2356. (Clone-specific: Flow cytometry). View Reference
-
Pizcueta P, Luscinskas FW. Monoclonal antibody blockade of L-selectin inhibits mononuclear leukocyte recruitment to inflammatory sites in vivo. Am J Pathol. 1994; 145(2):461-469. (Clone-specific: Flow cytometry, Immunohistochemistry). View Reference
-
Reichert RA, Jerabek L, Gallatin WM, Butcher EC, Weissman IL. Ontogeny of lymphocyte homing receptor expression in the mouse thymus. J Immunol. 1986; 136(10):3535-3542. (Clone-specific: Flow cytometry, Immunohistochemistry). View Reference
-
Reichert RA, Weissman IL, Butcher EC. Dual immunofluorescence studies of cortisone-induced thymic involution: evidence for a major cortical component to cortisone-resistant thymocytes. J Immunol. 1986; 136(10):3529-3534. (Clone-specific: Flow cytometry). View Reference
-
Reichert RA, Weissman IL, Butcher EC. Phenotypic analysis of thymocytes that express homing receptors for peripheral lymph nodes. J Immunol. 1986; 136(10):3521-3528. (Clone-specific: Flow cytometry). View Reference
-
Siegelman MH, Cheng IC, Weissman IL, Wakeland EK. The mouse lymph node homing receptor is identical with the lymphocyte cell surface marker Ly-22: role of the EGF domain in endothelial binding. Cell. 1990; 61(4):611-622. (Clone-specific: Blocking, Immunoprecipitation). View Reference
-
Vestweber D. Ligand-specificity of the selectins. J Cell Biochem. 1996; 61(4):585-591. (Biology). View Reference
-
Yang G, Mizuno MT, Hellstrom KE, Chen L. B7-negative versus B7-positive P815 tumor: differential requirements for priming of an antitumor immune response in lymph nodes. J Immunol. 1997; 158(2):851-858. (Clone-specific: Blocking). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.